Product Description
Bortezomib is the first therapeutic proteasome inhibitor to be tested in humans. It is approved in the U.S. for treating relapsed multiple myeloma and mantle cell lymphoma. In multiple myeloma, complete clinical responses have been obtained in patients with otherwise refractory or rapidly advancing disease.It was originally synthesized in 1995 at Myogenics and was approved in the United States by the FDA for use in multiple myeloma in May 2003.
We provide the API and the key intermediate (179324-87-9),white powder,
the purity of Bortezomib is more than 99.9% (HPLC), top quality, more competitive price.
Annual capacity 30kg.
Special packing is offered.

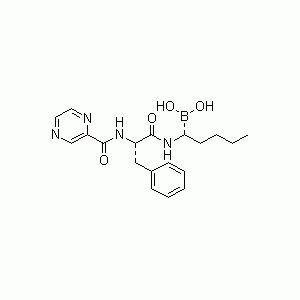



![132112-67-5, Bicyclo[2.2.1]heptane-2,6-dicarbonitrile](https://www.pudeepharm.com/wp-content/uploads/2024/05/675.jpg)
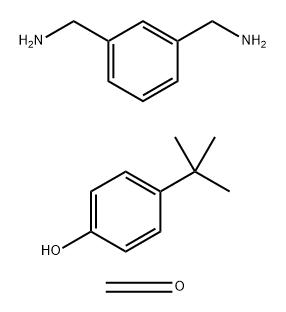
![189253-72-3, (2-{2-[(3-aminopropyl)(methyl)amino]ethoxy}ethyl)dimethylamine](https://www.pudeepharm.com/wp-content/uploads/2024/05/723.jpg)