Product Description
"
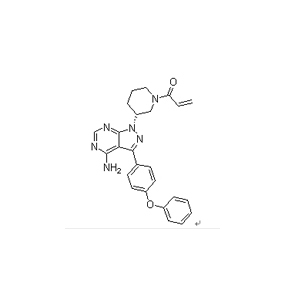
Ibrutinib is an anticancer drug targeting B-cell malignancies. It was approved by the US FDA on November 13, 2013 for the treatment of mantle cell lymphoma. On Feb. 12, 2014, the FDA expanded the approved use of ibrutinib to chronic lymphocytic leukemia (CLL). Further expansion to 17p deletion in CLL was approved 28 July 2014.
we provide API and Intermediats. Its appearance is white crystalline powder. the purity (HPLC) is more than 99% , top quality, more competitive.Special packing is offered.
"

![1803255-62-0,β-Ethoxy-4-[(2-methylphenyl)methoxy]-N-(methylsulfonyl)benzenepropanamide](https://www.pudeepharm.com/wp-content/uploads/2025/04/620.jpg)