Product Description
Nilotinib is a small-molecule tyrosine kinase inhibitor approved for the treatment of imatinib-resistant chronic myelogenous leukemia. it was developed based on the structure of the Abl-imatinib complex to address imatinib intolerance and resistance. Nilotinib is a selective Bcr-Abl kinase inhibitor that is 10-30 fold more potent than imatinib in inhibiting Bcr-Abl tyrosine kinase activity and proliferation of Bcr-Abl expressing cells.
It is approved by FDA- (29 October 2007), EMA- (29 September 2009), MHRA- (19 November 2007) and TGA- (17 January 2008) for use as a treatment for Philadelphia Chromosome (Ph+)-positive Chronic myelogenous leukaemia. on April 11, 2011, Novartis announced that it is discontinuing a Phase III trial of Tasigna® (nilotinib) for investigational use in the first-line treatment of gastrointestinal stromal tumor(GIST) based on the recommendation of an independent data monitoring committee.
We provide API and the key intermediate (641571-11-1) , white to off-white powder,
the purity of API is more than 99.5% (HPLC).
Top quality, more competitive price.
NMR,HPLC,IR,MOA,COA are available,
Special packing is offered.

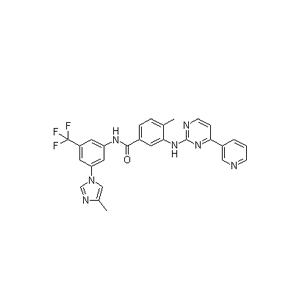



![132112-67-5, Bicyclo[2.2.1]heptane-2,6-dicarbonitrile](https://www.pudeepharm.com/wp-content/uploads/2024/05/675.jpg)
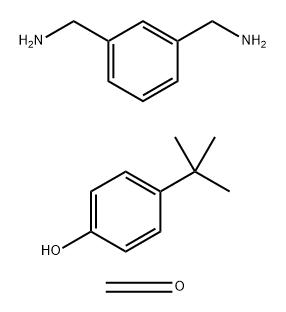
![189253-72-3, (2-{2-[(3-aminopropyl)(methyl)amino]ethoxy}ethyl)dimethylamine](https://www.pudeepharm.com/wp-content/uploads/2024/05/723.jpg)