Product Description
"
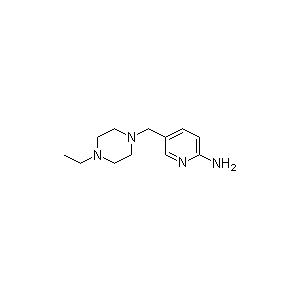
Abemaciclib is a drug for the treatment of advanced or metastatic breast cancers. It was developed by Eli Lilly. and it acts as a CDK inhibitor selective for CDK4 and CDK6.[1] It was designated as a breakthrough therapy by the U.S. Food and Drug Administration in October 2015.[2]In September 2017, it was approved for use in the United States by the Food and Drug Administration for "adult patients who have hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer that has progressed after taking therapy that alters a patient’s hormones"
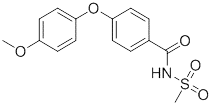
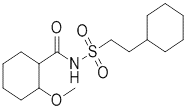
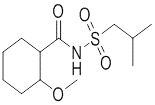
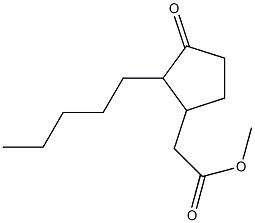
One of intermediates of Abemaciclib, the appearance is off-white solid, the purity is not less than 98% by HPLC.
"

![1311401-21-4,2-(2,2-Difluoro-1-oxopropoxy)-1-[(2,2-difluoro-1-oxopropoxy)methyl]ethyl 2-methyl-2-propenoate](https://www.pudeepharm.com/wp-content/uploads/2025/04/214.jpg)
![1311401-25-8,2-(2,2,3,3-Tetrafluoro-1-oxopropoxy)-1-[(2,2,3,3-tetrafluoro-1-oxopropoxy)methyl]ethyl 2-methyl-2-propenoate](https://www.pudeepharm.com/wp-content/uploads/2025/04/258.jpg)
![1803255-62-0,β-Ethoxy-4-[(2-methylphenyl)methoxy]-N-(methylsulfonyl)benzenepropanamide](https://www.pudeepharm.com/wp-content/uploads/2025/04/620.gif)