Product Description
"
Alectinib is an oral drug that blocks the activity of anaplastic lymphoma kinase (ALK) and is used to treat non-small-cell lung cancer (NSCLC). Alectinib was approved in Japan in July 2014 for the treatment of ALK fusion-gene positive, unresectable, advanced or recurrent non-small-cell lung cancer (NSCLC).
It was approved by the US Food and Drug Administration (FDA) in December 2015 to treat patients with advanced ALK-positive NSCLC whose disease worsened after, or who could not tolerate, treatment with crizotinib (Xalkori)
It got a conditional approval by the European Medicines Agency in February 2017 for the same indication. This means that additional studies are awaited to confirm a positive benefit-risk-ratio.
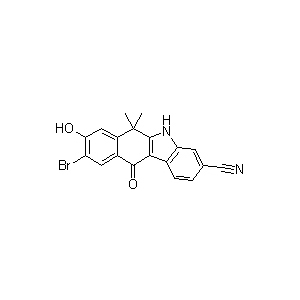
The material 9-Bromo-6,11-dihydro-8-hydroxy-6,6-dimethyl-11-oxo-5H-benzo[b]carbazole-3-carbonitrile(CAS Registry Number 1256579-06-2 ) is a key intermediate used for Alectinib.
"

![1311401-21-4,2-(2,2-Difluoro-1-oxopropoxy)-1-[(2,2-difluoro-1-oxopropoxy)methyl]ethyl 2-methyl-2-propenoate](https://www.pudeepharm.com/wp-content/uploads/2025/04/214.jpg)
![1311401-25-8,2-(2,2,3,3-Tetrafluoro-1-oxopropoxy)-1-[(2,2,3,3-tetrafluoro-1-oxopropoxy)methyl]ethyl 2-methyl-2-propenoate](https://www.pudeepharm.com/wp-content/uploads/2025/04/258.jpg)
![1803255-62-0,β-Ethoxy-4-[(2-methylphenyl)methoxy]-N-(methylsulfonyl)benzenepropanamide](https://www.pudeepharm.com/wp-content/uploads/2025/04/620.gif)