Product Description
"
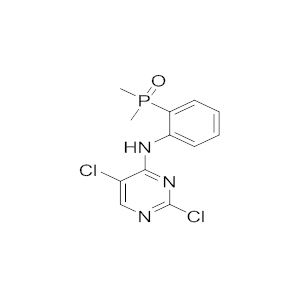
Brigatinib is an investigational small-molecule targeted cancer therapy. It acts as both a anaplastic lymphoma kinase (ALK) and epidermal growth factor receptor (EGFR) inhibitor.
In 28 April 2017, it was granted an Accelerated Approval from the US FDA for metastatic non-small cell lung cancer (NSCLC), as a 2nd-line therapy for ALK-positive NSCLC.
In 2016, Brigatinib was granted orphan drug status by the FDA for treatment of NSCLC.
Brigatinib could overcome resistance to osimertinib conferred by the EGFR C797S mutation if it is combined with an anti-EGFR antibody such as cetuximab or panitumumab.
One of intermediates of Brigatinib, the appearance is yellow solid, the purity is not less than 98% by HPLC.
"

![1311401-21-4,2-(2,2-Difluoro-1-oxopropoxy)-1-[(2,2-difluoro-1-oxopropoxy)methyl]ethyl 2-methyl-2-propenoate](https://www.pudeepharm.com/wp-content/uploads/2025/04/214.jpg)
![1311401-25-8,2-(2,2,3,3-Tetrafluoro-1-oxopropoxy)-1-[(2,2,3,3-tetrafluoro-1-oxopropoxy)methyl]ethyl 2-methyl-2-propenoate](https://www.pudeepharm.com/wp-content/uploads/2025/04/258.jpg)
![1803255-62-0,β-Ethoxy-4-[(2-methylphenyl)methoxy]-N-(methylsulfonyl)benzenepropanamide](https://www.pudeepharm.com/wp-content/uploads/2025/04/620.gif)